Quality of liver biopsy in ten hospital institutions of Bogota
DOI:
https://doi.org/10.22516/25007440.613Keywords:
Liver biopsy, quality of biopsy, definitive diagnososAbstract
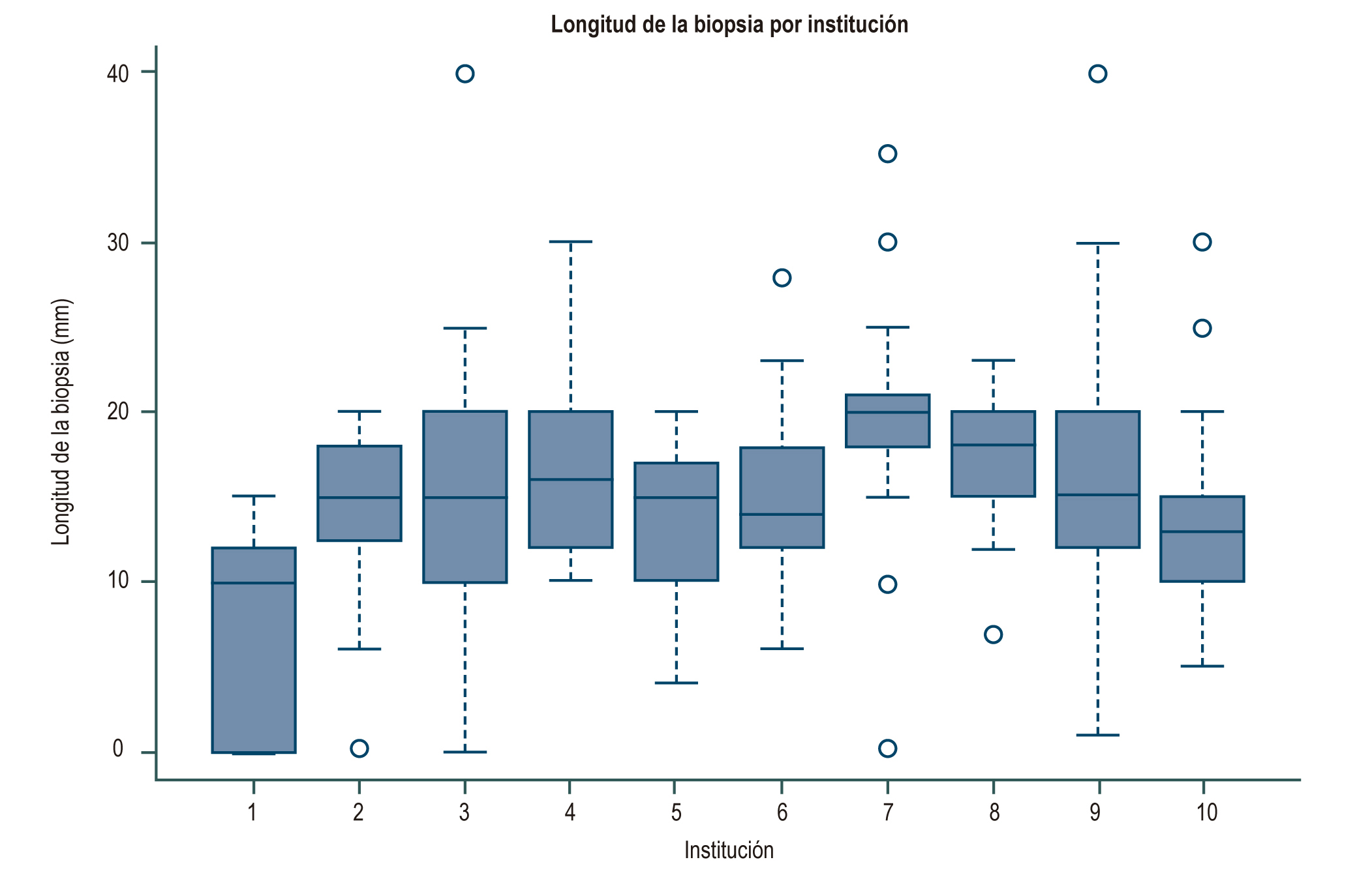
Introduction: Liver biopsy is the gold-standard for the diagnosis of diseases that compromise the liver, an adequate sample and an excellent report are elements that determine the usefulness of the test and the impact on decision-making. Objective: To assess the quality of liver biopsies based on the frequency of a “definitive diagnosis” in their report and their relationship to the number of portal spaces and their reported length. Materials and methods: Retrospective observational study based on registry, from January 1, 2010 to July 30, 2017. Liver biopsy, review of medical records and evaluation of the pathology result were performed. Results: 659 pathology reports from 10 institutions were included. The percentage of reporting of portal spaces varied between 15% and 87.4%. The median biopsy length was 15 mm (RIQ 10-20) and the median number of portal spaces was 10 (RIQ 7-15). The definitive diagnoses were between 35% and 69%, probable diagnoses between 25% and 63% and without diagnosis between 5% and 31.8%. In the result of the logistic regression of the diagnosis, it was found that the number of portal spaces presented an OR of 1.10 (95% CI 1.04-1.17) and length OR 1.76 (1.10-2.82). Conclusions: In Bogotá there are 3 institutions with a diagnostic performance in liver biopsy report above 60%. The definitive diagnosis in liver biopsy was associated in this study with the presence of a cylinder of liver tissue of adequate length and number of portal spaces.
Downloads
References
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49(3):1017-44. https://doi.org/10.1002/hep.22742
Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50(1):1-3. https://doi.org/10.1016/j.jhep.2008.10.014
Poynard T, Benhamou Y, Thabut D, Ratziu V. Liver biopsy: the best standard...when everything else fails. J Hepatol. 2009;50(6):1267-8. https://doi.org/10.1016/j.jhep.2009.02.010
Tapper EB, Lok ASF. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377(8):756-768. https://doi.org/10.1056/NEJMra1610570
Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol. 2020;36(3):184-191. https://doi.org/10.1097/MOG.0000000000000621
Klein, M.A. Diagnostic Liver Pathology. By R.G. Lee, 517 pp. St. Louis: Mosby, 1994. Hepatology, 20: 1645-1646. https://doi.org/10.1002/hep.1840200644
Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495-500. https://doi.org/10.1056/NEJM200102153440706
Schiano TD, Azeem S, Bodian CA, Bodenheimer HC Jr, Merati S, Thung SN, et al. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3(9):930-5. https://doi.org/10.1016/s1542-3565(05)00541-0
Cholongitas E, Quaglia A, Samonakis D, Senzolo M, Triantos C, Patch D, et al. Transjugular liver biopsy: how good is it for accurate histological interpretation? Gut. 2006;55(12):1789-94. https://doi.org/10.1136/gut.2005.090415
Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39(2):239-44. https://doi.org/10.1016/s0168-8278(03)00191-0
Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69(8):1382-1403. https://doi.org/10.1136/gutjnl-2020-321299
Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125(5):710-21. https://doi.org/10.1309/W3XC-NT4H-KFBN-2G0B
Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC; Trent Hepatitis C Study Group. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53(3):451-5. https://doi.org/10.1136/gut.2003.021691
Coral GP, Antunes AD, Serafini AP, Araujo FB, Mattos AA. Liver biopsy: importance of specimen size in the diagnosis and staging of chronic viral hepatitis. Rev Inst Med Trop Sao Paulo. 2016;58:10. https://doi.org/10.1590/S1678-9946201658010
Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449-57. https://doi.org/10.1016/j.hep.2003.09.022
Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28(2):323-31. https://doi.org/10.1002/hep.510280206
Röcken C, Meier H, Klauck S, Wolff S, Malfertheiner P, Roessner A. Large-needle biopsy versus thin-needle biopsy in diagnostic pathology of liver diseases. Liver. 2001;21(6):391-7. https://doi.org/10.1034/j.1600-0676.2001.210605.x
Cholongitas E, Quaglia A, Samonakis D, Mela M, Patch D, Dhillon AP, et al. Transjugular liver biopsy in patients with diffuse liver disease: comparison of three cores with one or two cores for accurate histological interpretation. Liver Int. 2007;27(5):646-53. https://doi.org/10.1111/j.1478-3231.2007.01496.x
Sporea I, Gherhardt D, Popescu A, Sirli R, Cornianu M, Herman D, et al. Does the size of the needle influence the number of portal tracts obtained through percutaneous liver biopsy? Ann Hepatol. 2012;11(5):691-5. https://doi.org/10.1016/S1665-2681(19)31444-9
Chi H, Hansen BE, Tang WY, Schouten JN, Sprengers D, Taimr P, et al. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol. 2017;29(1):36-41. https://doi.org/10.1097/MEG.0000000000000731
Midia M, Odedra D, Shuster A, Midia R, Muir J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: a scoping review. Diagn Interv Radiol. 2019;25(1):71-80. https://doi.org/10.5152/dir.2018.17525
Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194(3):784-9. https://doi.org/10.2214/AJR.08.2122
Bejarano PA, Koehler A, Sherman KE. Second opinion pathology in liver biopsy interpretation. Am J Gastroenterol. 2001;96(11):3158-64. https://doi.org/10.1111/j.1572-0241.2001.05273.x
Hahm GK, Niemann TH, Lucas JG, Frankel WL. The value of second opinion in gastrointestinal and liver pathology. Arch Pathol Lab Med. 2001;125(6):736-9. https://doi.org/10.1043/0003-9985(2001)125<0736:TVOSOI>2.0.CO;2
Tomaszewski JE, Bear HD, Connally JA, Epstein JI, Feldman M, Foucar K, et al. Consensus conference on second opinions in diagnostic anatomic pathology. Who, What, and When. Am J Clin Pathol. 2000;114(3):329-35. https://doi.org/10.1093/ajcp/114.3.329
Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898-906. https://doi.org/10.1053/j.gastro.2005.03.084
Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614-8. https://doi.org/10.1111/j.1572-0241.2002.06038.x
Soloway RD, Baggenstoss AH, Schoenfield LJ, Summerskill WH. Observer error and sampling variability tested in evaluation of hepatitis and cirrhosis by liver biopsy. Am J Dig Dis. 1971;16(12):1082-6. https://doi.org/10.1007/BF02235164
Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanu M, et al. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25(6):733-9. https://doi.org/10.1111/j.1365-2036.2007.03252.x
Downloads
Published
How to Cite
Issue
Section
License
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.