KRAS Gene Mutation in Patients Undergoing Liver Resections for Colorectal Cancer. Is There an Advantage to Anatomical Resections?
DOI:
https://doi.org/10.22516/25007440.929Keywords:
Colorectal cancer, Liver metastases, Metastasectomy, KRAS mutationAbstract
Introduction: Several factors have been described to make a prognostic assessment of patients with liver metastases due to colorectal cancer and to define the benefit of the surgical management of metastatic involvement; one of these factors is the status of the KRAS gene since its mutation is associated with worse outcomes. This study aims to describe the outcomes for a retrospective series of patients after liver resections for metastatic colorectal cancer concerning KRAS gene status.
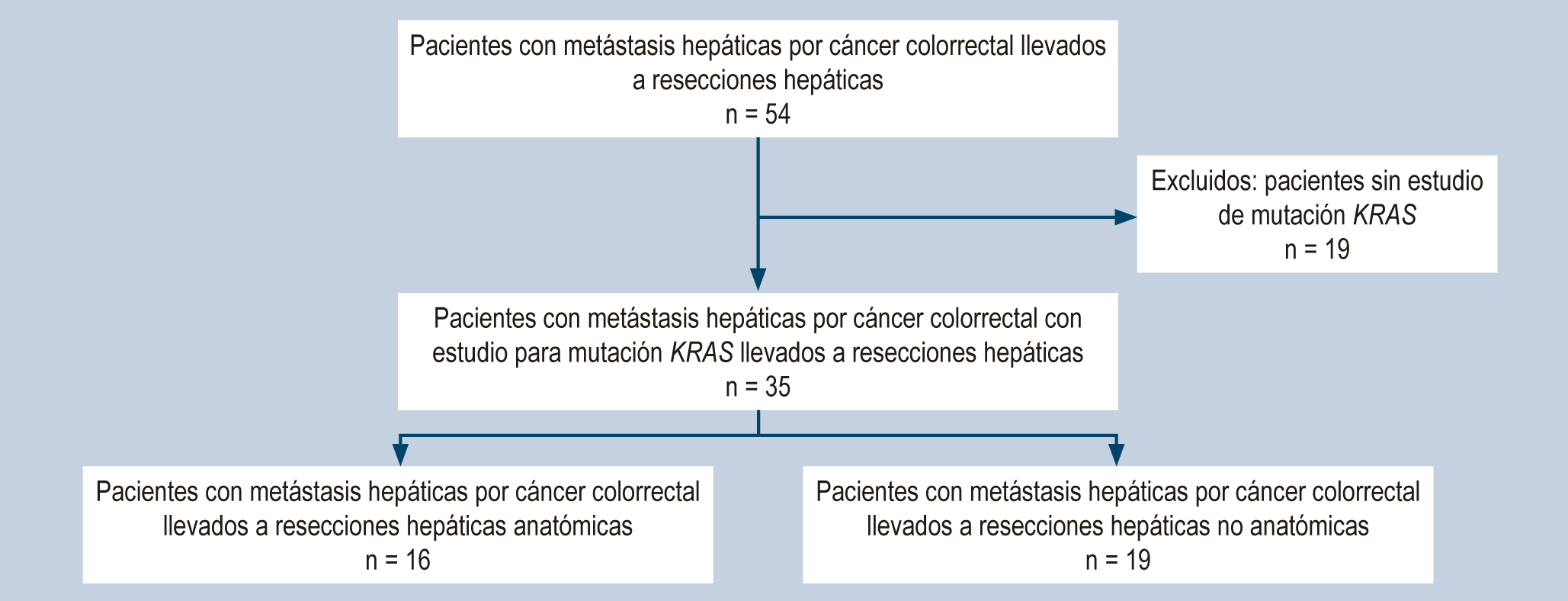
Materials and methods: The study involves a retrospective cohort of patients undergoing liver metastasectomy for colorectal cancer with KRAS mutation study from 2009-2013 at the National Institute of Cancerology in Colombia. Five-year survival analyses (overall and disease-free) were performed according to KRAS mutation status and the type of liver resection performed using the Kaplan-Meier estimate.
Results: 35 patients undergoing liver metastasectomy were analyzed, of which 42.8% had KRAS gene mutation. Median overall survival was 34.2 months for patients with KRAS- mutant and 46.5 for non-mutant. The median survival for KRAS-mutant patients with anatomic resections was 43.5 months versus 23.5 months for nonanatomic resections.
Conclusions: Performing anatomic resections during liver metastasectomy in patients with KRAS mutants could be associated with an improvement in overall survival. It is necessary to continue building the evidence for adequate decision-making in patients with KRAS mutants who will undergo liver resections.
Downloads
References
Cancer Today. Data visualization tools for exploring the global cancer burden in 2020 [Internet]. Lyon: IARC; 2020 [consultado el 16 de octubre de 2020]. Disponible en: https://gco.iarc.fr/today
Jones RP, Jackson R, Dunne DF, Malik HZ, Fenwick SW, Poston GJ, et al. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg. 2012;99(4):477-86. https://doi.org/10.1002/bjs.8667
Passiglia F, Bronte G, Bazan V, Galvano A, Vincenzi B, Russo A. Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;99:150-7. https://doi.org/10.1016/j.critrevonc.2015.12.015
Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301.
https://doi.org/10.2147/CLEP.S34285
Adam R, Vinet E. Regional treatment of metastasis: Surgery of colorectal liver metastases. Ann Oncol. 2004;15(Suppl 4):103-106. https://doi.org/10.1093/annonc/mdh912
Chow FCL, Chok KSH. Colorectal liver metastases: An update on multidisciplinary approach. World J Hepatol. 2019;11(2):150-172. https://doi.org/10.4254/wjh.v11.i2.150
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-18. https://doi.org/10.1097/00000658-199909000-00004
Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA Jr, Donehower RC, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119(23):4137-44.
https://doi.org/10.1002/cncr.28347
Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175-83. https://doi.org/10.1002/bjs.9870
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg. 2016;263(1):146-52. https://doi.org/10.1097/SLA.0000000000001194
Gold JS, Are C, Kornprat P, Jarnagin WR, Gönen M, Fong Y, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247(1):109-17. https://doi.org/10.1097/SLA.0b013e3181557e47
Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262(3):476-85. https://doi.org/10.1097/SLA.0000000000001427
Cady B, Jenkins RL, Steele GD Jr, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227(4):566-71. https://doi.org/10.1097/00000658-199804000-00019
Margonis GA, Sasaki K, Andreatos N, Kim Y, Merath K, Wagner D, et al. KRAS Mutation Status Dictates Optimal Surgical Margin Width in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2017;24(1):264-271.
https://doi.org/10.1245/s10434-016-5609-1
Bronte G, Rolfo C, Peeters M, Russo A. How to find the Ariadne’s thread in the labyrinth of salvage treatment options for metastatic colorectal cancer? Expert Opin Biol Ther. 2014;14(6):743-8. https://doi.org/10.1517/14712598.2014.902926
Nagashima I, Takada T, Nagawa H, Muto T, Okinaga K. Proposal of a new and simple staging system of colorectal liver metastasis. World J Gastroenterol. 2006;12(43):6961-5. https://doi.org/10.3748/wjg.v12.i43.6961
Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466-75. https://doi.org/10.1016/j.ejca.2012.02.057
Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51(10):1243-52. https://doi.org/10.1016/j.ejca.2015.04.007
Rizzo S, Bronte G, Fanale D, Corsini L, Silvestris N, Santini D, et al. Prognostic vs predictive molecular biomarkers in colorectal cancer: is KRAS and BRAF wild type status required for anti-EGFR therapy? Cancer Treat Rev. 2010;36 Suppl 3:S56-61. https://doi.org/10.1016/S0305-7372(10)70021-9
Knijn N, Mekenkamp LJ, Klomp M, Vink-Börger ME, Tol J, Teerenstra S, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104(6):1020-6.
https://doi.org/10.1038/bjc.2011.26
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643-8. https://doi.org/10.1158/0008-5472.CAN-06-4158
Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120(24):3965-71. https://doi.org/10.1002/cncr.28954
Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015;112(12):1921-8.
https://doi.org/10.1038/bjc.2015.142
Stremitzer S, Stift J, Gruenberger B, Tamandl D, Aschacher T, Wolf B, et al. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99(11):1575-82. https://doi.org/10.1002/bjs.8909
Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619-26.
https://doi.org/10.1097/SLA.0b013e3182a5025a
Margonis GA, Buettner S, Andreatos N, Sasaki K, Ijzermans JNM, van Vugt JLA, et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann Surg. 2017;266(4):641-649.
https://doi.org/10.1097/SLA.0000000000002367
Choi M, Han DH, Choi JS, Choi GH. Can the presence of KRAS mutations guide the type of liver resection during simultaneous resection of colorectal liver metastasis? Ann Hepatobiliary Pancreat Surg. 2022;26(2):125-132. https://doi.org/10.14701/ahbps.21-127
Kawai T, Ishii T, Uchida Y, Sato A, Naito S, Kitaguchi K, et al. Impact of anatomical liver resection on patient survival in KRAS-wildtype colorectal liver metastasis: A multicenter retrospective study; Surgery. 2022;172(4):1133-1140. https://doi.org/10.1016/j.surg.2022.05.014
Teng H-W, Huang Y-C, Lin J-K, Chen W-S, Lin T-C, Jiang J-K, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106(2):123-129. https://doi.org/10.1002/jso.23063
Tosi F, Magni E, Amatu A, Mauri G, Bencardino K, Truini M, et al. Effect of KRAS and BRAF mutations on survival of metastatic colorectal cancer after liver resection: a systematic review and meta-analysis. Clin Colorectal Cancer. 2017;16(3):e153-e163. https://doi.org/10.1016/j.clcc.2017.01.004
Javed S, Benoist S, Devos P, Truant S, Guimbaud R, Lièvre A, et al. Prognostic factors of BRAF V600E colorectal cancer with liver metastases: a retrospective multicentric study. World J Surg Oncol. 2022;20(1):131. https://doi.org/10.1186/s12957-022-02594-2
Johnson B, Jin Z, Truty MJ, Smoot RL, Nagorney DM, Kendrick ML, et al. Impact of metastasectomy in the multimodality approach for BRAF V600E metastatic colorectal cancer: the mayo clinic experience. Oncologist. 2018;23(1):128-134.
https://doi.org/10.1634/theoncologist.2017-0230
Gagnière J, Dupré A, Gholami SS, Pezet D, Boerner T, Gönen M, et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann Surg. 2020;271(1):147-154. https://doi.org/10.1097/SLA.0000000000002968
Zhang Q, Peng J, Ye M, Weng W, Tan C, Ni S, et al. KRAS Mutation Predicted More Mirometastases and Closer Resection Margins in Patients with Colorectal Cancer Liver Metastases. Ann Surg Oncol. 2020;27(4):1164-1173. https://doi.org/10.1245/s10434-019-08065-5
Margonis GA, Sasaki K, Kim Y, Samaha M, Buettner S, Amini N, et al. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2016;20(11):1821-1829. https://doi.org/10.1007/s11605-016-3198-8
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.



















