The behavior of liver diseases in a cohort of Colombian patients with COVID-19
DOI:
https://doi.org/10.22516/25007440.853Keywords:
Mortality, Fatty liver, SARS Virus, Cirrhosis of the liverAbstract
Introduction: Severe acute respiratory syndrome type 2 coronavirus infection (SARS-CoV-2) is receiving the most attention now. The asymptomatic elevation of transaminases is typical in the liver, and liver involvement varies from 14 % to 78 %. The assessment of liver comorbidities is scarce, with prevalence ranging between 2 % and 11 %.
Aim: To describe the behavior of a cohort of patients with liver diseases who fell ill with coronavirus disease 2019 (COVID-19).
Materials and methods: This retrospective observational study analyzed the behavior of a cohort of patients with liver diseases who fell ill with COVID-19.
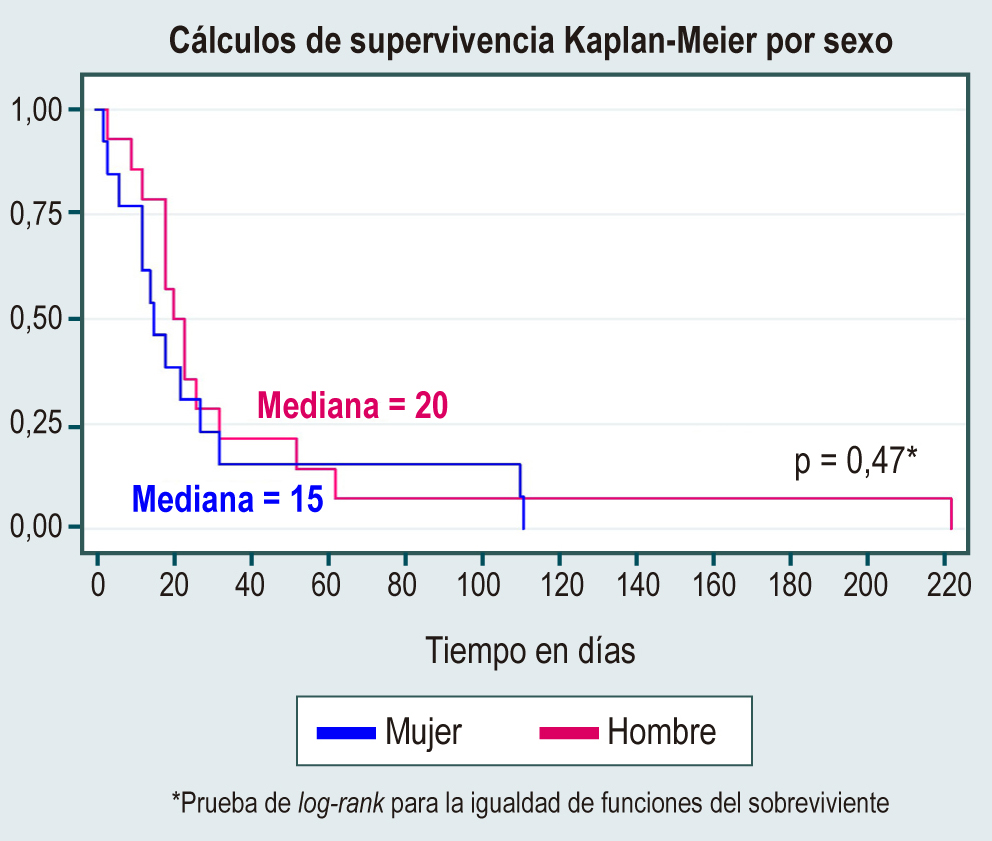
Results: 543 patients became ill with COVID-19, of which 300 were women (55.3 %). The median age at diagnosis of liver disease was 52 years. The leading causes of liver disease were nonalcoholic steatohepatitis (49.5 %), cholestatic disease (7.7 %), and hepatitis C and B viruses (6.3 %). Alanine aminotransferase (ALT) had a median of 52 U/L (interquartile range [IQR]: 30–98) and aspartate aminotransferase (AST) 32 U/L (IQR: 23–62). Mortality due to viral infection was 5.7 %, with an incidence rate of 2.9 (95 % confidence interval [CI]: 2–4.2).
Conclusions: It is a retrospective study but, until the preparation of the manuscript, it had been the first cohort in Colombia to describe the behavior of liver diseases in patients who become ill with COVID-19. No statistically significant differences were found between the causes of liver disease that confer a higher risk of mortality; however, having decompensated cirrhosis is the only condition related to mortality.
Downloads
References
Perisetti A, Gajendran M, Mann R, Elhanafi S, Goyal H. COVID-19 extrapulmonary illness - special gastrointestinal and hepatic considerations. Dis Mon. 2020;66(9):101064. https://doi.org/10.1016/j.disamonth.2020.101064
Huang C , Wang Y , Li X , Ren L , Zhao J , Hu Y , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020;395(10223):497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
Jin X , Lian JS , Hu JH , Gao J , Zheng L , Zhang YM , et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut . 2020 .
Fan Z , Chen L , Li J , Cheng X , Jingmao Y , Tian C , et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol . 2020. https://doi.org/10.1101/2020.02.26.20026971
Dorrell RD, Dougherty MK, Barash EL, Lichtig AE, Clayton SB, Jensen ET. Gastrointestinal and hepatic manifestations of COVID-19: A systematic review and meta-analysis (published online ahead of print, 2020 Nov 21). JGH Open. 2020;5(1):107-115. https://doi.org/10.1002/jgh3.12456
Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now?. Arab J Gastroenterol. 2020;21(1):3-8. https://doi.org/10.1016/j.ajg.2020.03.002
Hoffmann M, Kleine-Weber H, Krüger N, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-1 coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020.01.31.929042; https://doi.org/10.1101/2020.01.31.929042
Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020; published online Feb 4. https://doi.org/10.1101/2020.02.03.931766.
Zhang Chao, Shi Lei, Wang Fu-Sheng. Liver injury in COVID-19: management and challenges. Lancet gastroenterol Hepatol 2020. https://doi.org/10.1016/S2468-1253(20)30057-1.
Kim KD, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med 2007;13:1248-52. https://doi.org/10.1038/nm1633.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507- 13. https://doi.org/10.1016/S0140-6736(20)30211-7
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708-1720. https://doi.org/10.1056/NEJMoa2002032
Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425-434. https://doi.org/10.1016/S1473-3099(20)30086-4
Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730-1741. https://doi.org/10.1111/all.14238
Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
Surveillance Epidemiology of Coronavirus (COViD-19) Under Research Exclusion. SECURECirrhosis, 2020. Disponible en: https://covidcirrhosis.web.unc.edu/
COVID-Hep. COVID-Hep.net: Coronavirus (COVID-19) in liver disease reporting registry, 2020. Disponible en: https://www.covid-hep.net/
Calleri A, Saracco M, Pittaluga F, Cavallo R, Romagnoli R, Martini S. Seroconversion After Coronavirus Disease 2019 Vaccination in Patients Awaiting Liver Transplantation: Fact or Fancy?. Liver Transpl. 2022;28(2):180-187. https://doi.org/10.1002/lt.26312
Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435-438. https://doi.org/10.1016/j.jhep.2021.04.020
Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol 2020; 73(2):451- 453. https://doi.org/10.1016/j.jhep.2020.03.044
Zhou YJ, Zheng KI, Wang XB, et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol 2020; 73(3):719-721. https://doi.org/10.1016/j.jhep.2020.04.027
Ponziani FR, Del Zompo F, Nesci A, et al. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2 positive patients. Aliment Pharmacol Ther 2020;10.1111/apt.15996. https://doi.org/10.1111/apt.15996
Singh S, Khan A. Clinical Characteristics and Outcomes of Corona¬virus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020;S0016-5085(20)30585-0. doi:10.1053/j. gastro.2020.04.064
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista colombiana de Gastroenterología

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.



















