Case report of glucosamine-chondroitin induced hepatotoxicity in a public hospital in Lima, Peru
DOI:
https://doi.org/10.22516/25007440.325Keywords:
Glucosamine, chondroitin, toxicity, transaminases, osteoarthritisAbstract
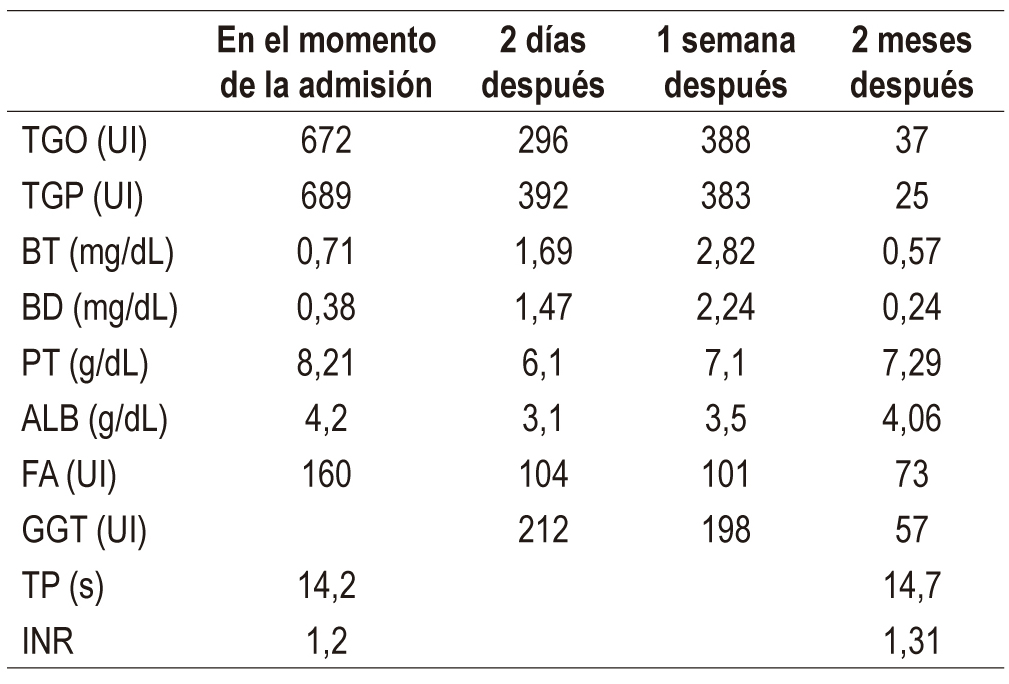
The human body naturally produces glucosamine and chondroitin which are important components of the cartilaginous system. There are multiple clinical indications for them as medicines, but they are primarily used for osteoarthritis. Hepatotoxicity induced by these biomolecules is uncommon, and the only reports in the world literature are isolated individual cases. This article presents the case of a patient with glucosamine-chondroitin-induced hepatocellular damage who was admitted to the hospital with respiratory symptoms and malaise. Marked hypertransaminemia was found in laboratory tests. Etiologies such as alcohol, viral hepatitis and autoimmune liver diseases were ruled out, and abdominal ultrasound found no evidence of chronic liver disease. Discontinuance of glucosamine and chondroitin led to a considerable decrease in hypertransaminemia after one week with total improvement two months of hospital discharge. This case adds to the small number reported worldwide and is relevant for future systematic studies to clarify the outlook for this disease.
Downloads
References
Ministerio de Salud. Informe técnico N° 22-2007: glucosamina sulfato 1,5 g/condroitina 1,2 g (polvo). Lima: Dirección General de Medicamentos, Insumos y Drogas; 2007.
Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75(1):37-44. http://dx.doi.org/10.1136/annrheumdis-2014-206792
Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459-64. http://dx.doi.org/10.1136/ard.2009.120469
Smith A, Dillon J. Acute liver injury associated with the use of herbal preparations containing glucosamine: three case studies. BMJ Case Rep. 2009;2009. pii: bcr02.2009.1603. https://doi.org/10.1136/bcr.02.2009.1603
Ebrahim V, Albeldawi M, Chiang DJ. Acute liver injury associated with glucosamine dietary supplement. BMJ Case Rep. 2012;2012. pii: bcr2012007665. https://doi.org/10.1136/bcr-2012-007665
Yang L, Aronsohn A, Hart J, Jensen D. Herbal hepatoxicity from Chinese skullcap: A case report. World J Hepatol. 2012;4(7):231–233. https://doi.org/10.4254/wjh.v4.i7.231
Linnebur SA, Rapacchietta OC, Vejar M. Hepatotoxicity associated with chinese skullcap contained in Move Free Advanced dietary supplement: two case reports and review of the literature. Pharmacotherapy. 2010;30(7):750, 258e-262e. https://doi.org/10.1592/phco.30.7.750
Ossendza RA, Grandval P, Chinoune F, Rocher F, Chapel F, Bernardini D. Acute cholestatic hepatitis due to glucosamine forte. Gastroenterol Clin Biol. 2007;31(4):449-50. https://doi.org/10.1016/S0399-8320(07)89410-3
Ip S, Jeong R, Schaeffer DF, Yoshida EM. Unusual case of drug-induced cholestasis due to glucosamine and chondroitin sulfate. World J Hepatol. 2015;7(24):2559–2562. https://doi.org/10.4254/wjh.v7.i24.2559
Cerda C, Bruguera M, Parés A. Hepatotoxicity associated with glucosamine and chondroitin sulfate in patients with chronic liver disease. World J Gastroenterol. 2013;19(32):5381-4. https://doi.org/10.3748/wjg.v19.i32.5381
Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomized placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74(5):851-8. https://doi.org/10.1136/annrheumdis-2013-203954
Bruyère O, Altman RD, Reginster JY. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S12-7. https://doi.org/10.1016/j.semarthrit.2015.11.011
Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808. https://doi.org/10.1056/NEJMoa052771
de Vos BC, Landsmeer MLA, van Middelkoop M, Oei EHG, Krul M, Bierma-Zeinstra SMA, et al. Long-term effects of a lifestyle intervention and oral glucosamine sulphate in primary care on incident knee OA in overweight women. Rheumatology (Oxford). 2017;56(8):1326-1334. https://doi.org/10.1093/rheumatology/kex145
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Glucosamine. [actualizada el 12 de marzo de 2020]. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK547949/
Downloads
Published
How to Cite
Issue
Section
License
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.


| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |