Type 1 Refractory Celiac Disease: Report of Two Cases with Different Therapeutic Approaches
DOI:
https://doi.org/10.22516/25007440.1271Keywords:
Celiac disease, Treatment, Diagnosis, gluten-free diet, budesonide, azathioprineAbstract
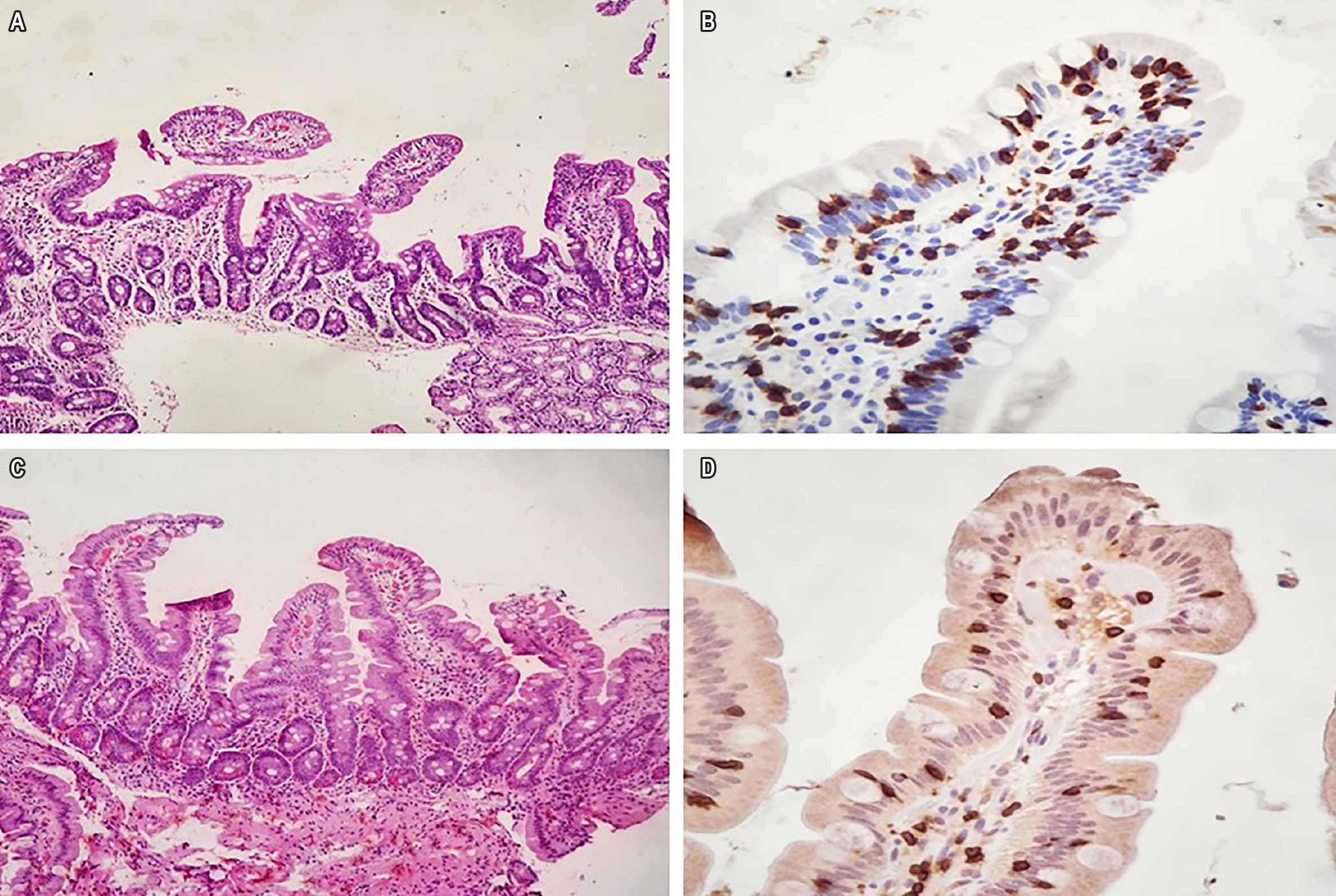
Background: Lifelong strict adherence to a gluten-free diet (GFD) is the effective treatment for celiac disease (CD), leading to symptom remission and mucosal healing. Refractory celiac disease (RCD) is defined as the persistence or relapse of symptoms and intestinal damage in individuals previously diagnosed with CD after at least 12 months of strict GFD adherence, occurring in a minority of CD patients. Diagnosis and differentiation of RCD type are performed via specific immunohistochemistry on duodenal biopsy.
Case 1: A 52-year-old male with a prior CD diagnosis presented with persistent symptoms even after five years of strict GFD adherence. He was diagnosed with type 1 refractory RCD and treated with oral budesonide (9 mg/day for 8 months), achieving clinical remission, with normalization of duodenal mucosal histopathology.
Case 2: A 62-year-old female with a prior CD diagnosis and two years of strict GFD adherence presented with severe symptoms. She was diagnosed with type 1 RCD and treated with azathioprine at 2 mg/kg/day for 24 months, resulting in complete symptom remission and restoration of duodenal mucosal integrity.
Conclusions: In addition to strict adherence to a healthy gluten-free diet, both oral budesonide and azathioprine were effective in treating type 1 RCD, as patients achieved and maintained clinical remission without drug-related adverse effects. Histological response, demonstrating complete normalization of duodenal mucosal architecture, confirmed the success of therapy.
Downloads
References
Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7(5):583-613. https://doi.org/10.1177/2050640619844125
Green PHR, Paski S, Ko CW, Rubio-Tapia A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology. 2022;163(5):1461-1469. https://doi.org/10.1053/j.gastro.2022.07.086
Rubio-Tapia A, Hill ID, Semrad C, Kelly CP, Greer KB, Limketkai BN, et al. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am J Gastroenterol. 2023;118(1):59-76. https://doi.org/10.14309/ajg.0000000000002075
Tye-Din JA. Review article: Follow-up of coeliac disease. Aliment Pharmacol Ther. 2022;56 Suppl 1(Suppl 1):S49-S63. https://doi.org/10.1111/apt.16847
Silvester JA, Therrien A, Kelly CP. Celiac Disease: Fallacies and Facts. Am J Gastroenterol. 2021;116(6):1148-1155. https://doi.org/10.14309/ajg.0000000000001218
Volta U, Caio G, De Giorgio R. Mistakes in refractory coeliac disease and how to avoid them. UEG Education. 2019;19:15–18.
Penny HA, Baggus EMR, Rej A, Snowden JA, Sanders DS. Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients. 2020;12(1):216. https://doi.org/10.3390/nu12010216
Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59(4):547-57. https://doi.org/10.1136/gut.2009.195131
van Wanrooij RL, Müller DM, Neefjes-Borst EA, Meijer J, Koudstaal LG, Heideman DA, et al. Optimal strategies to identify aberrant intra-epithelial lymphocytes in refractory coeliac disease. J Clin Immunol. 2014;34(7):828-35. https://doi.org/10.1007/s10875-014-0075-7
Goerres MS, Meijer JW, Wahab PJ, Kerckhaert JA, Groenen PJ, Van Krieken JH, et al. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment Pharmacol Ther. 2003;18(5):487-94. https://doi.org/10.1046/j.1365-2036.2003.01687.x
Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136(1):81-90. https://doi.org/10.1053/j.gastro.2008.09.069
Soldera J, Salgado K, Pêgas KL. Refractory celiac disease type 2: how to diagnose and treat? Rev Assoc Med Bras (1992). 2021;67(2):168-172. https://doi.org/10.1590/1806-9282.67.02.20200618
Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-76; quiz 677. https://doi.org/10.1038/ajg.2013.79
Marsh MN. Mucosal pathology in gluten sensitivity. En: Marsh MN (editor). Coeliac disease. Oxford: Blackwell Scientific Publications; 1992. p. 136-991.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11(10):1185-94. https://doi.org/10.1097/00042737-199910000-00019
Patey-Mariaud De Serre N, Cellier C, Jabri B, Delabesse E, Verkarre V, Roche B, et al. Distinction between coeliac disease and refractory sprue: a simple immunohistochemical method. Histopathology. 2000;37(1):70-7. https://doi.org/10.1046/j.1365-2559.2000.00926.x
Aziz M, Haghbin H, Khan RS, Khan Z, Weissman S, Kamal F, et al. Celiac Disease Is Associated with Microscopic Colitis in Refractory Cases in Adults: A Systematic Review and Meta-Analysis of Observational Studies. Dig Dis Sci. 2022;67(8):3529-3542. https://doi.org/10.1007/s10620-021-07232-7
Demiroren K. Possible relationship between refractory celiac disease and malignancies. World J Clin Oncol. 2022;13(3):200-208. https://doi.org/10.5306/wjco.v13.i3.200
Packova B, Kohout P, Dastych M, Prokesova J, Grolich T, Kroupa R. Malignant complications of celiac disease: a case series and review of the literature. J Med Case Rep. 2022;16(1):460. https://doi.org/10.1186/s13256-022-03682-3
Mukewar SS, Sharma A, Rubio-Tapia A, Wu TT, Jabri B, Murray JA. Open-Capsule Budesonide for Refractory Celiac Disease Am J Gastroenterol. 2017;112(6):959-967. https://doi.org/10.1038/ajg.2017.71
Edsbäcker S, Bengtsson B, Larsson P, Lundin P, Nilsson A, Ulmius J, et al. A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment Pharmacol Ther. 2003;17(4):525-36. https://doi.org/10.1046/j.1365-2036.2003.01426.x
Tack GJ, van Asseldonk DP, van Wanrooij RL, van Bodegraven AA, Mulder CJ. Tioguanine in the treatment of refractory coeliac disease--a single centre experience. Aliment Pharmacol Ther. 2012;36(3):274-81. https://doi.org/10.1111/j.1365-2036.2012.05154.x
Iqbal U, Chaudhary A, Karim MA, Anwar H, Merrell N. Refractory celiac disease successfully treated with azathioprine. Gastroenterology Res. 2017;10(3):199-201. https://doi.org/10.14740/gr819w
Jiang C, Barkin JA, Barkin JS. Exocrine Pancreatic Insufficiency Is Common in Celiac Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2023;68(8):3421-3427. https://doi.org/10.1007/s10620-023-07965-7
Leonard MM, Cureton P, Fasano A. Indications and Use of the Gluten Contamination Elimination Diet for Patients with Non-Responsive Celiac Disease. Nutrients. 2017;9(10):1129. https://doi.org/10.3390/nu9101129
Raiteri A, Granito A, Giamperoli A, Catenaro T, Negrini G, Tovoli F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J Gastroenterol. 2022;28(1):154-175. https://doi.org/10.3748/wjg.v28.i1.154
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista colombiana de Gastroenterología

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.



















