Drug-Induced Pancreatitis Due to Deferasirox: A Case Report
DOI:
https://doi.org/10.22516/25007440.1211Keywords:
Pancreatitis, Acute pancreatitis, Abdominal pain, Deferasirox, ExjadeAbstract
Abstract
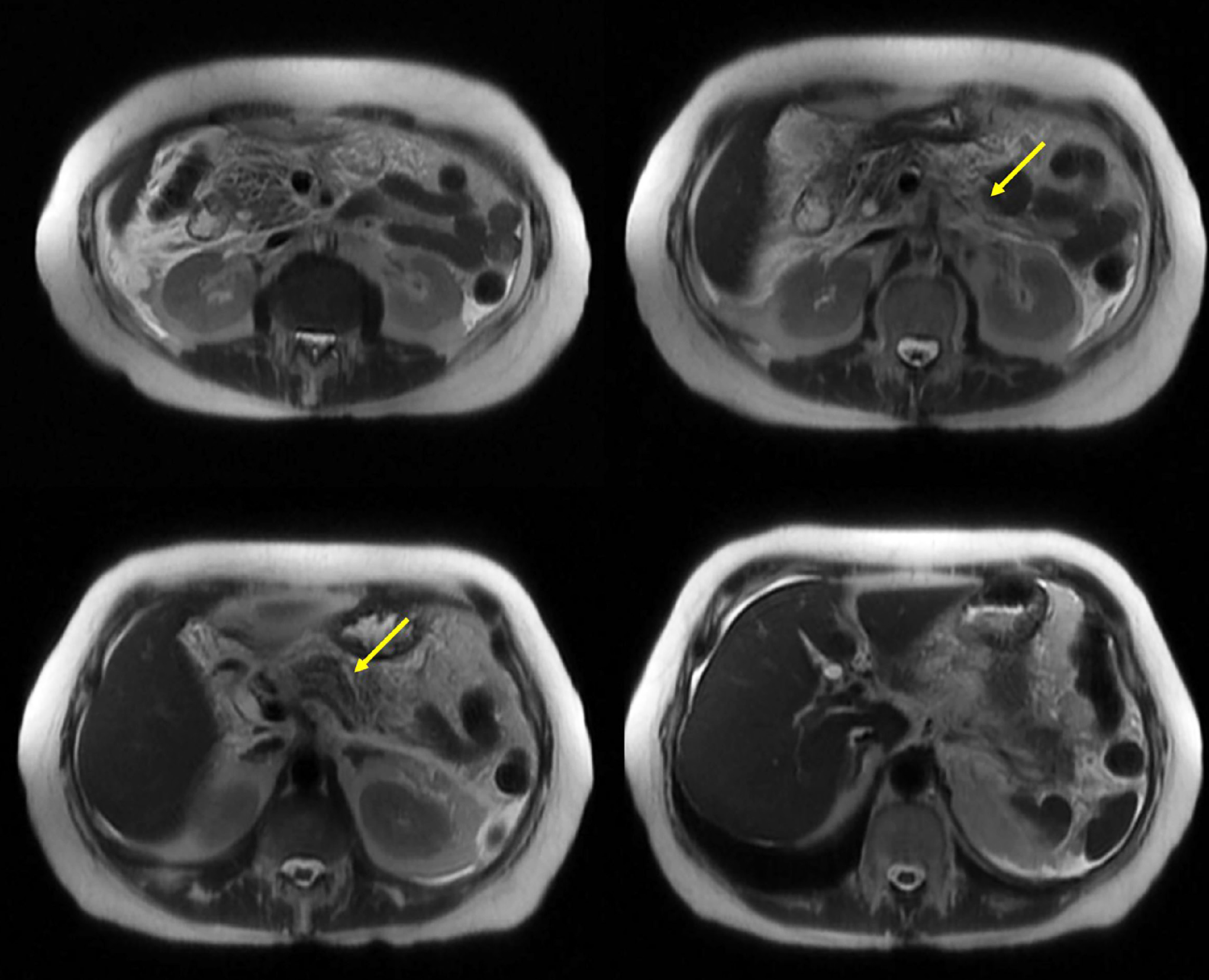
Pancreatitis, or inflammation of the pancreas, is a common reason for medical consultation, particularly in emergency departments when patients present with abdominal pain. It represents a significant financial burden on healthcare systems, as one in three cases progresses to moderate or severe pancreatitis, leading to increased morbidity, mortality, and complications. Currently, the primary causes of pancreatitis include obstructive biliary and non-biliary factors, resulting from reflux and the absence of enzyme flow into the intestine. This leads to enzyme activation within the pancreatic tissue, causing self-digestion. Alcohol consumption and hypertriglyceridemia contribute to cellular toxicity due to the metabolic breakdown of these substances. Drug-induced pancreatitis is a rare condition associated with antibiotics, analgesics, and antidepressants. Deferasirox, an iron chelator primarily used in patients requiring frequent blood transfusions to prevent iron overload, has rarely been linked to pancreatitis. However, available data suggest a possible correlation, and the pharmaceutical manufacturer lists pancreatitis as a potential adverse effect in the drug’s technical specifications. This report presents the case of a 71-year-old female patient who developed moderate to severe pancreatitis without major complications. Physicians ruled out the most common causes of pancreatitis and concluded that the triggering factor was the iron-chelating agent deferasirox.
Downloads
References
Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156(7):1951-1968.e1. https://doi.org/10.1053/j.gastro.2018.11.081
Yadav D, Lowenfels AB. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology. 2013;144(6):1252-61. https://doi.org/10.1053/j.gastro.2013.01.068
Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156(1):254-272.e11. https://doi.org/10.1053/j.gastro.2018.08.063
Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis. JAMA. 2021;325(4):382. https://doi.org/10.1001/jama.2020.20317
Muñoz D, Medina R, Botache WF, Arrieta RE. Pancreatitis aguda: puntos clave. Revisión argumentativa de la literatura. Revista Colombiana de Cirugía. 2023;38:339-351. https://doi.org/10.30944/20117582.2206
Rojas CA, Salazar Otoya N, Sepúlveda Copete M, Maldonado Gutiérrez C, Castro Llanos AM, Gómez Córdoba Y, et al. Características clínicas de pacientes con pancreatitis aguda atendidos en un hospital de alta complejidad en Cali. Rev Colomb Gastroenterol. 2021;36(3):341-8. https://doi.org/10.22516/25007440.682
Kundumadam S, Fogel EL, Gromski MA. Gallstone pancreatitis: general clinical approach and the role of endoscopic retrograde cholangiopancreatography. Korean J Intern Med. 2021;36(1):25-31. https://doi.org/10.3904/kjim.2020.537
Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca 2+ -dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63(8):1313-24. https://doi.org/10.1136/gutjnl-2012-304058
Curto C, Caillard C, Desurmont T, Sebag F, Brunaud L, Kraimps JL, et al. Pancréatite aiguë et hyperparathyroïdie primaire: étude multicentrique de l’Association francophone de chirurgie endocrinienne. J Chir (Paris). 2009;146(3):270–4. https://doi.org/10.1016/j.jchir.2009.06.016
Kabil MF, Nasr M. Deferasirox: A comprehensive drug profile. Profiles Drug Subst Excip Relat Methodol. 2024;49:1-18. https://doi.org/10.1016/bs.podrm.2023.11.001
Stumpf JL. Deferasirox. American Journal of Health-System Pharmacy. 2007;64(6):606–16. https://doi.org/10.2146/ajhp060405
Nisbet-Brown E, Olivieri NF, Giardina PJ, Grady RW, Neufeld EJ, Séchaud R, et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361(9369):1597–602. https://doi.org/10.1016/S0140-6736(03)13309-0
Cappellini MD. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107(9):3455–62. https://doi.org/10.1182/blood-2005-08-3430
Vichinsky E, Bernaudin F, Forni GL, Gardner R, Hassell K, Heeney MM, et al. Long‐term safety and efficacy of deferasirox (Exjade®) for up to 5 years in transfusional iron‐overloaded patients with sickle cell disease. Br J Haematol. 2011;154(3):387–97. https://doi.org/10.1111/j.1365-2141.2011.08720.x
European Medicines Agency. Ficha técnica o resumen de las características del producto. European Medicines Agency; 2008 [consultado el 17 de febrero de 2024]. Disponible en: https://ec.europa.eu/health/documents/community-register/2019/20190719145377/anx_145377_es.pdf
Health Sciences Authority. Risk of pancreatitis associated with the use of deferasirox in paediatric patients [Internet]. HSA; 2015 [consultado el 17 de febrero de 2024]. Disponible en: https://www.hsa.gov.sg/announcements/safety-alert/risk-of-pancreatitis-associated-with-the-use-of-deferasirox-in-paediatric-patients
Novartis. Jadenu® (deferasirox) Handbook. Singapore: Novartis; 2021 [consultado el 17 de febrero de 2024]. Disponible en: https://www.hsa.gov.sg/docs/default-source/hprg-vcb/pem-pmg-pac/jadenu_pmg_sg2305221982_17052021_adminupdate_22052023.pdf
Departamento de Farmacovigilancia. Informe anual. Buenos Aires: Departamento de Farmacovigilancia, Administración Nacional de Medicamentos Alimentos y Tecnología Médica; 2017 [consultado el 20 de febrero de 2024]. Disponible en: https://www.argentina.gob.ar/sites/default/files/farmacovigilancia-informe_2017.pdf
Ministerio de Salud. Disposición 3363-16 [Internet]. Buenos Aires; Ministerio de Salud, Secretaría de Políticas, Regulación e Institutos; 2016 [consultado el 20 de febrero de 2024]. Disponible en: https://boletin.anmat.gob.ar/Abril_2016/Dispo_3363-16.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista colombiana de Gastroenterología

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.



















